HemCon ® Bandage PRO

Acute trauma wounds
- • Amputations
- • Blast injuries
- • Lacerations
- • Gunshot and Stab Wounds
- • Motor Vehicle Accidents
- • Trauma
Wound Debridement
- • Diabetic Foot Ulcers
- • Pressure Ulcers (partial and full thickness)
- • Venous Ulcers
- • Surgical Site Incisions
Positive Outcomes. STAT.
HEMOSTATIC BANDAGE FOR OOZING TO SEVERE BLEEDING CONTROL IN TRAUMA AND WOUND CARE
HemCon Bandage PRO is ideally suited for quick bleeding control in a variety of external wound types and is easily administered with current protocol of dressing and direct pressure.
• Cost effective:
Lower cost than other alternatives; standardizes the number of SKUs ordered. Rapid bleeding control can result in less need for cauterization.
• Fast Hemostasis:
Stops bleeding from oozing to severe arterial in minutes¹˒²˒³; minimizes blood loss. Reduced blood loss can reduce the need for transfusions.
• Dependable:
Maintains structural integrity that won't crack, crumble or shed in wounds; creates a strong clot; provides localized support of clotting.
• Easy to Use:
Reduces direct pressure protocol to free up staff; intuitive application requires limited training; addresses a variety of wound types.
• Safe:
Works independently of the clotting cascade; provides an antibacterial barrier against 24 microorganisms including MRSA, VRE, A. baumannii and C. difficile; no known contra-indications.
INDICATION FOR USE
The HemCon Bandage PRO is a hemostatic dressing for the external, temporary control of severely bleeding wounds, intended for emergency use. The HemCon Bandage PRO also controls bleeding in patients following hemodialysis and is indicated for the control of bleeding from the skin at percutaneous needle access, vascular access, and percutaneous catheter access sites.
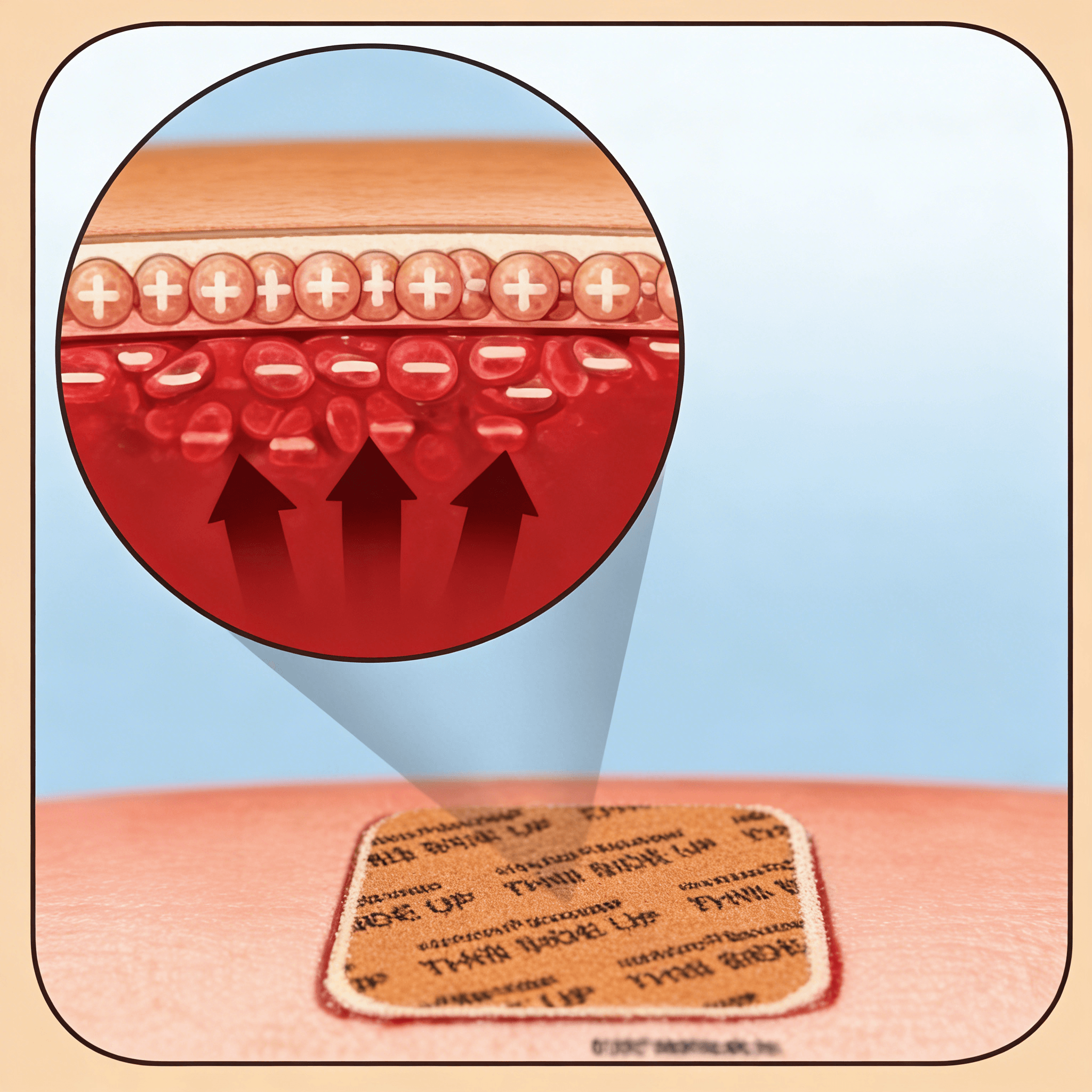
HOW HEMCON BANDAGES WORK
HemCon products are made from chitosan, a naturally occurring polysaccharide. Chitosan is positively charged, attracting negatively-charged red blood cells and platelets. This ionic interaction creates a supportive, primary seal at the wound site independent to the clotting cascade to control all degrees of bleeding.
APPLICATION GUIDE
- Apply HemCon Bandage PRO directly over actively bleeding wound with tan backing away from wound. If necessary, the bandage can be cut or folded to the size of the wound.
- Hold pressure until bleeding is controlled.
- Apply outer bandage wrap (not included) to secure dressing on wound site, if required.
- Recheck the wound for potential bleeding as necessary. If hemostasis is not achieved or for recurrent bleeding, remove patch with saline or water and re-apply a new patch until hemostasis is achieved.
REMOVAL INSTRUCTIONS
- The HemCon Bandage PRO can remain in place for up to 48 hours and should be removed with water or saline.
REDUCTION OF MICROORGANISMS
HemCon Bandage PRO was tested for reduction of microorganisms against the following species. The log reduction data demonstrates the antibacterial barrier effect.
| Organism | Gram Stain | Log Reduction |
|---|---|---|
| Escherichia coli ATCC 8739 | - | >5.2 |
| Klebsiella pneumoniae ATCC 4352 | - | >5.3 |
| Streptococcus pyogenes ATCC 19615 | + | >5.0 |
| Staphylococcus aureus (MRSA) ATCC 33591 | + | >4.5 |
| Staphylococcus epidermidis ATCC 12228 | + | >5.2 |
| Salmonella choleraesuis ATCC 10708 | - | >5.1 |
| Pseudomonas aeruginosa ATCC 27853 | - | >4.3 |
| Enterococcus faecalis (VRE) ATCC 51299 | + | >5.4 |
| Enterobacter aerogenes ATCC 13048 | - | >5.4 |
| Serratia marcescens ATCC 13880 | - | >5.0 |
| Shigella flexneri ATCC 12022 | - | >5.1 |
| Streptococcus mutans ATCC 25175 | + | >3.2 |
| Clostridium difficile ATCC 9689 | + | >5.5 |
| Streptococcus pneumoniae ATCC 49619 | + | 5.8 |
| Shigella species ATCC 11966 | - | >5.4 |
| Enterobacter aerogenes ATCC 13048 | - | >5.0 |
| Proteus mirabilis ATCC 43071 | - | >3.2 |
| Proteus vulgaris ATCC 13315 | - | >4.8 |
| Citrobacter freundii ATCC 8090 | - | >4.3 |
| Acinetobacter baumannii ATCC 13001 | - | >4.2 |
| Moraxella catarrhalis ATCC 25240 | - | >4.1 |
| Micrococcus luteus ATCC 49732 | + | 4.9 |
| Vibrio cholerae ATCC 11558 | - | >4.9 |
ORDER INFORMATION
| Product Name | Size | Part Number | Configuration |
|---|---|---|---|
| HemCon Bandage PRO | 4in x 4in | 1003 | 5/bx, 100/cs |
FDA 510(K): K080818
Tax ID: 81-2091181
MMF-225 rev 4 / US/04/18
